Primary Difference Between a Primary and Continuous Cell Culture

Imagine that you would like to plant a flower garden. You can look for flowers that perfectly fit in your environment, although they may be expensive and require expert care. Or you can choose varieties that perhaps are not ideal, but which grow everywhere, are affordable and do not need much care. Similarly, when you are starting a research project and need to decide whether to use human primary cells or immortal cell lines, you have to consider a range of factors.
Do you want to build a cell culture model that closely represents the human in vivo situation? In this case, human primary cells are a better choice. Also, if you plan to publish your results, you should keep in mind that reviewers might want you to validate your results in a system that is relevant for human physiology. Or do you want a model that is easy to work with and well established? Here, immortal cell lines would be preferred, although the cells might not behave exactly as they do in humans.
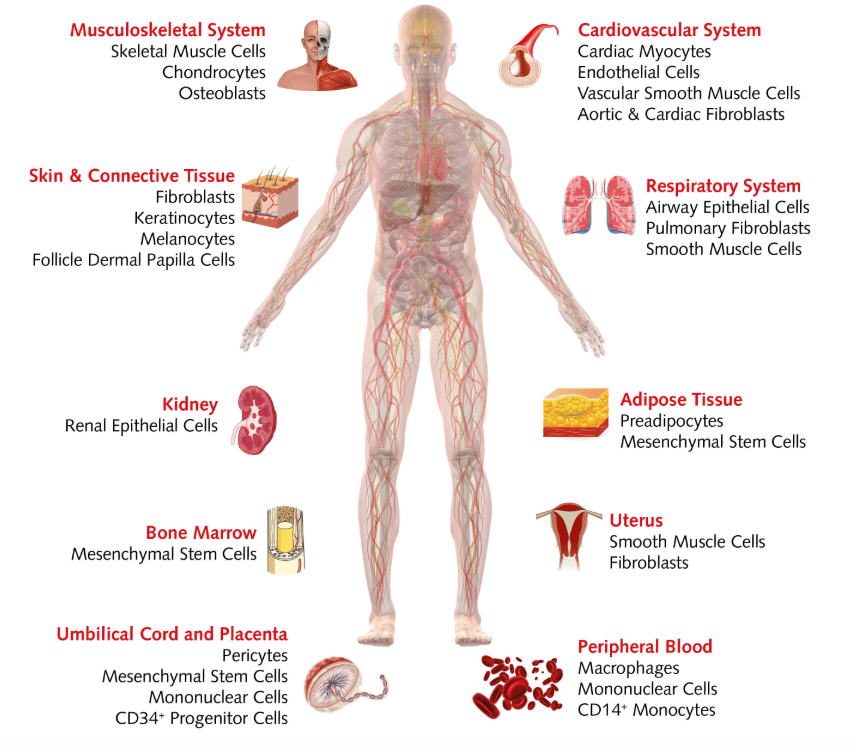
A wide range of choices:
HLA-typed human primary cells
Human primary cells: peeking into real life
Human primary cells are isolated directly from tissues and retain the morphological and functional characteristics of their tissue of origin. For example, original tumor tissue from colorectal cancer preserves several tumor markers and known microRNAs. In comparison, cell lines display differences in their expression (Pastor et al., 2010).
Primary cells, however, don't live forever. They undergo senescence processes and have limited potential for self-renewal and differentiation. As they age, they show morphological and functional changes, which is why you should use them in early passages. The genetic characteristics and age of the donors are also important, as their cells can behave differently under the same culture conditions. This can be a good thing when you are investigating how specific cellular features differ across the population or when testing new compounds. On the other hand, do your tests require low variance? Then use cells from the same donor. One advantage of human primary cells is that you needn't rely on animal models. This means you can avoid inter-species differences – such as in anatomy, molecular pathways and metabolism – which can affect drug toxicity and mode of action.
How you handle primary cells determines success
Human primary cell cultures can be initiated from healthy cells or cancer cells. They come from healthy donors, organ donation, surgical specimens, fetal tissues or post-mortem donors. When planning your experiments, keep in mind that the source of human primary cells is limited. This means you might not be able to get extra material from the same donor. Also, primary cultures derived from tissue explants are a mix of different cells at different stages, so if you want to initiate more homogeneous cultures, you might need to purify specific cell types. Because primary cells are more sensitive than cell lines, they often require additional nutrients and growth factors.
Because different types of cells need different media to grow and survive, always optimize culture conditions for each cell type. Cell lines need high levels of serum. However, serum is not standardized and various lots can display high variability of the many substances contained. This could greatly influence the results of your research and even make them inconsistent. In the case of human primary cells, remember that high levels of antibiotics decrease their viability and growth. As cells reach the different stages of culture, you need to characterize them and identify any morphological and functional changes. This is relevant for quality control. Of course, good cell culture practice demands that you document all the information necessary to track the materials and methods. In this way, you ensure that your model is transparent and reproducible (Pamies et al., 2018).
Fighting endotoxin contamination in cell cultures
Contamination threatens cell culture studies. Not only bacteria, fungi, yeast and mycoplasma are the culprits, endotoxins also cause problems. They affect cellular growth and function in vitro as well as in vivo (Ryan et al, 2018). Endotoxins are lipopolysaccharides derived from the outer membrane of most gram-negative bacteria, which are stable even at high temperatures. Endotoxins often contaminate labware, as they show high affinity for hydrophobic materials such as plastic. Sources of contamination include water used for washing, media and sera, additives, laboratory plastics and glassware. You can detect endotoxins with the limulus amebocyte lysate (LAL) assay, as LAL forms quantifiable gel-clots in their presence.
Various cells types react differently to the presence of endotoxins, and endotoxin limits should be set for each cell type in in vitro cultures (Nomura et al., 2017). To minimize the risk of endotoxin contamination, use certified nonpyrogenic media, sera, and labware, and check the water used in the lab, especially when working with sensitive cells.
Cell lines: an integral part of biological research
 Working with primary cells rests upon decades of research using cell lines. Since the early 20th century, cell lines have provided scientists with great insights into the biological processes of human cells. Immortal cell lines have become a powerful tool for countless applications including testing drug metabolism and cytotoxicity, studying gene function, as well as producing vaccines, antibodies and biological compounds.
Working with primary cells rests upon decades of research using cell lines. Since the early 20th century, cell lines have provided scientists with great insights into the biological processes of human cells. Immortal cell lines have become a powerful tool for countless applications including testing drug metabolism and cytotoxicity, studying gene function, as well as producing vaccines, antibodies and biological compounds. As mentioned, the aim of your research determines the type of cell culture you use. Researchers who plan to investigate basic biological processes, manipulate cellular functions, establish new methods or perform preliminary screenings tend to choose immortalized cell lines. Why? They are cost effective, easy to work with, and can be kept in culture for longer periods of time. Cell lines are also easy to manipulate and expand. (Kaur et al., 2012). Is high throughput screening on the agenda? Here, the unlimited supply of material could also be an advantage.

Yet, even if experimental findings obtained with cell lines can be clearly interpreted and consistently compared with previous studies, keep in mind that the physiological relevance of these studies might not be very high. There are also limits to what you can do with cell lines. In fact, some show only marginal similarity to the original primary cells, as serial passages can cause variations in genotype and phenotype. You might end up with an altered genomic content and an abnormal expression profile. Cell lines lack key morphological or functional features, so they might not be able to induce relevant biomarkers. This means that the results you obtained with cell lines in the lab cannot be fully translated to humans. Over longer periods of time, immortal cell lines can be also contaminated by other cells or microorganisms (see blog post: Mycoplasma Contamination – Small Organisms Cause Big Trouble). So please take this advice: Before doing any work with cell lines, always validate them first to make sure your cells are not misidentified or contaminated (Lorsch et al, 2014).
In short, base your choice of human primary cells or immortal cell lines on your research aim. In many cases, immortal cell lines offer a very valuable model for preliminary experiments. Then use human primary cells to replicate key findings. This increases translation and makes your results more relevant to human physiology.

Primary cells and cell lines in drug development
Have a look at our scientific poster and find out how to use
- human primary cells and
- immortal cell lines
in your drug development project.
Read about human cell-based assays, cytotoxicity testing and much more.
Download our poster
campanelliforelut.blogspot.com
Source: https://promocell.com/in-the-lab/human-primary-cells-and-immortal-cell-lines/
Belum ada Komentar untuk "Primary Difference Between a Primary and Continuous Cell Culture"
Posting Komentar